Profile
ORCID ID: 0000-0001-6050-2860
I am a Biochemist by training and obtained my degree at the Technical University of Munich, Germany, in 2007 (BSc and MSc in Biochemistry). I then undertook a PhD (with summa cum laude) in Pharmacology at the Department for Molecular Medicine, Max Planck Institute of Biochemistry in Munich. My project focused on the Epidermal bullosa subtype Kindler Syndrome, exploring the role of the focal adhesion protein Kindlin-1 in skin development, homeostasis and skin cancer. For the research achievements of my PhD I was awarded the Junior Research Award of the Max Planck Institute and Young Investigator Award of the German Matrix Biology Society.
To extend my research track record in skin cell biology I joined the lab of Prof. Fiona Watt (Centre for Stem Cell and Regenerative Medicine, KCL) for my Postdoc in 2014 on an EMBO-long-term fellowship. My postdoctoral research focused on dissecting the plasticity and tissue scale behaviour of skin dermal fibroblast lineages during development, homoeostasis and wound healing. In 2018 I was awarded an EMBO advanced fellowship enabling me to expand my current research and transition to an independent position. I joined the Department of Endocrinology at the WHRI as a lecturer in 2019.
Research
Group members
Abubkr Abdelaal Ahmed (PhD) and Thomas Kirk (PhD)
Summary

Figure 1: Skin dermis. Fibroblast lineage distribution in the distinct de. rmal sublayers (papillary, reticular, DWAT). Lineages give also rise to dermal sheath (DS), dermal papilla (DP) and arrector pili muscle (APM). Fb, fibroblast
The skin is our largest organ and most important protective barrier. It is constantly exposed to damage caused by injuries or environmental stress such as UV light-induced sunburns. Skin repair requires coordinated function of two layers, the epidermis and dermis and perturbation of this process is associated with multiple skin diseases, ranging from fibrosis to cancer. The epidermis is a multilayered (stratified) epithelium that is separated by a basement membrane from the underlying dermis (Figure 1). The dermis forms the skin scaffold consisting of a dense extracellular matrix (ECM) meshwork and different cell populations, including fibroblasts, sensory neurons, and endothelial and immune cells. During dermal development multipotent fibroblasts differentiate into distinct subpopulations that create the dermal sublayers, papillary, reticular and dermal white adipose tissue (DWAT). These fibroblast subpopulations differ in location and function and their cell identity and composition changes with age. This dermal maturation is governed by a tight balance of fibroblast proliferation, quiescence and ECM deposition. Our recent findings revealed that there is a coordinated switch in fibroblast behaviour of highly proliferative in embryonic development to quiescence postnatally allowing efficient ECM deposition/remodelling, which is necessary and sufficient to define dermal architecture.
While this postnatal quiescent state can be maintained long-term in postnatal skin, upon wounding different fibroblast lineages become activated at the wound site. These α-smooth-muscle-actin-(aSMA)-positive myofibroblasts quickly resume proliferation and migrate into the wound bed. Besides depositing/remodelling ECM in the wound bed, fibroblasts show astonishing plasticity and are able to acquire a dermal papilla or adipocyte fate in response to distinct signals promoting hair follicle and DWAT regeneration. After tissue repair, wound bed fibroblasts re-establish a quiescent state to maintain skin homeostasis. Deregulation of these complex developmental processes in the dermis is associated with several skin pathologies, including fibrosis, chronic wounds and cancer.
Thus despite the fundamental role of fibroblasts in tissue maintenance and diseases, the extrinsic and intrinsic regulatory mechanisms controlling dermal fibroblast lineages behaviour and fate are largely unknown. In the lab we are pursuing a multidisciplinary approach to address the following key research questions:
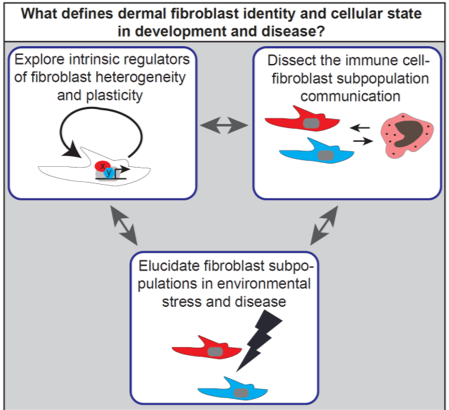
How are fibroblast lineages specified and maintained throughout life?
Which immune cell subtypes regulate fibroblast lineage behaviour and how are they influencing each other during skin development, regeneration and ageing?
What are the pathogenic processes promoting and maintaining aberrant fibroblast function in disease conditions?
To answer these questions our lab is combining genome-wide screening, proteomics, in silico analysis, in vivo imaging, lineage tracing of dermal cells with multiple cell biology techniques. Our goal is to elucidate the fundamental biology defining fibroblast identity and plasticity during development, regeneration and disease paving the way for new treatment strategies targeting pathological fibroblast behaviour in skin diseases and potentially other organs.

Figure 2: Fibroblast subpopulations during wound healing. (A) Exploring why skin regeneration ability decreases with age. There is a strong Wnt/β-catenin signalling activation (TOPGFP, green) in adult wound beds and placodes of regenerating hair follicles in neonatal wounds. In addition neonatal wounds show higher abundance of papillary fibroblasts (Lrig1, red) compared to reticular fibroblasts (Sca1, blue), which has strong implications for tissue regeneration. (B) In vivo live imaging of dermal fibroblast subpopulations during wound healing revealed how fibroblasts (nuclei, green; cytoplasm, red) polarise and migrate towards the wound bed centre in the early tissue repair phase.

Figure 3: Investigating fibroblast collagen deposition and remodelling during skin development and disease. (A) Collagen fibres (red) start to appear in reticular dermis during embryonic development and collagen signal increases with age. (B) Fibrotic (diseased) skin is characterised by strong immune cell infiltration (CD45, white) and increased collagen deposition/remodelling.




